How Sapphire Valve Disc for Medical Use Extends Device Lifespan Longer
Sapphire Valve Disc For Medical Use is UPCERA's focused answer to the industry's demand for longer-lasting, safer fluid control in critical care devices. We engineer each disc from sapphire (alpha-alumina single crystal) and finish it to medical-grade tolerances. The result is steady sealing, less wear, and fewer maintenance interruptions over the life of the device.

The Real Cost of Valve Wear - and Why Lifespan Matters
Healthcare teams need dependable valves. Downtime disrupts therapy. Replacements add cost and risk. Many legacy valve discs face three pain points: abrasive media that scores surfaces, harsh sterilization cycles that stress materials, and cleaning agents that attack chemistry. Micro-leaks emerge, friction rises, and service intervals shorten.
At UPCERA, we designed the Sapphire Valve Disc For Medical Use to address these issues at the material level. Sapphire's exceptional hardness (Mohs 9, second only to diamond) keeps the sealing face smooth, resisting grooves that trigger leakage. Its chemical inertness prevents swelling, softening, or surface reactions that undermine seal integrity. Extreme temperature stability from −200 °C to 2000 °C tolerates rapid thermal cycling during sterilization without distortion. In practice, valves stay calibrated longer, and critical flow accuracy is preserved where polymers or metals often struggle.
Hidden costs compound when wear sets in. A slight increase in breakaway torque can skew dosing or flow profiles, forcing recalibration. Unplanned service interrupts clinical schedules and increases spare inventory needs. Cleaning becomes more frequent as surfaces roughen, and each cycle adds wear. Over time, this turns into higher total cost of ownership and measurable clinical risk.
✅ Less unplanned maintenance: Stable surfaces cut emergency service calls.
✅ Steady performance: Consistent sealing reduces drift, protecting dosing accuracy.
✅ Better sterilization tolerance: Geometry and finish hold through repeated cycles.
• The Material Gap in Legacy Designs
Traditional metals can corrode or gall under mixed lubrication, creating particulate and scoring. Many polymers creep under load or shed particles during abrasion, and elastomers may swell in aggressive cleaners. These changes raise friction, increase actuation energy, and accelerate leakage.
By contrast, sapphire combines wear resistance, chemical neutrality, electrical insulation, optical transparency (UV to IR), and radiation resistance. This blend helps maintain low, predictable friction over time, even near high-energy equipment or in rigorous cleaning regimes. For medical devices, that means a Sapphire Valve Disc For Medical Use keeps its shape and surface, sustaining seal integrity and flow accuracy across long service intervals - translating directly into safer therapy and lower lifecycle cost.
How UPCERA Sapphire Extends Service Life in the Field
At UPCERA, we pair the right material with the right process. Sapphire is hard - and that makes machining challenging. We use specialized equipment and CNC processes to achieve flatness, parallelism, and edge quality that keep the seal consistent across millions of cycles. Fewer defects mean fewer starting points for wear.
Sapphire's unique profile brings multiple life-extension levers together:
✅ Hardness that resists abrasion: Mohs 9 mitigates scoring from particulates and throttling.
✅ Extreme temperature stability: −200 °C to 2000 °C helps discs survive rapid sterilization and thermal shocks.
✅ Chemical inertness: Cleaning agents and corrosive media do not degrade the surface or dimensions.
✅ Electrical insulation & radiation resistance: Supports reliability in devices exposed to energy sources or imaging environments.
✅ Optical transparency (UV to IR): Enables in-line sensing or visual inspection where designers need it.
These advantages reduce cumulative damage, so torque remains steady and sealing load stays predictable. That is how a wear-resistant medical valve disc translates material science into longer device life.
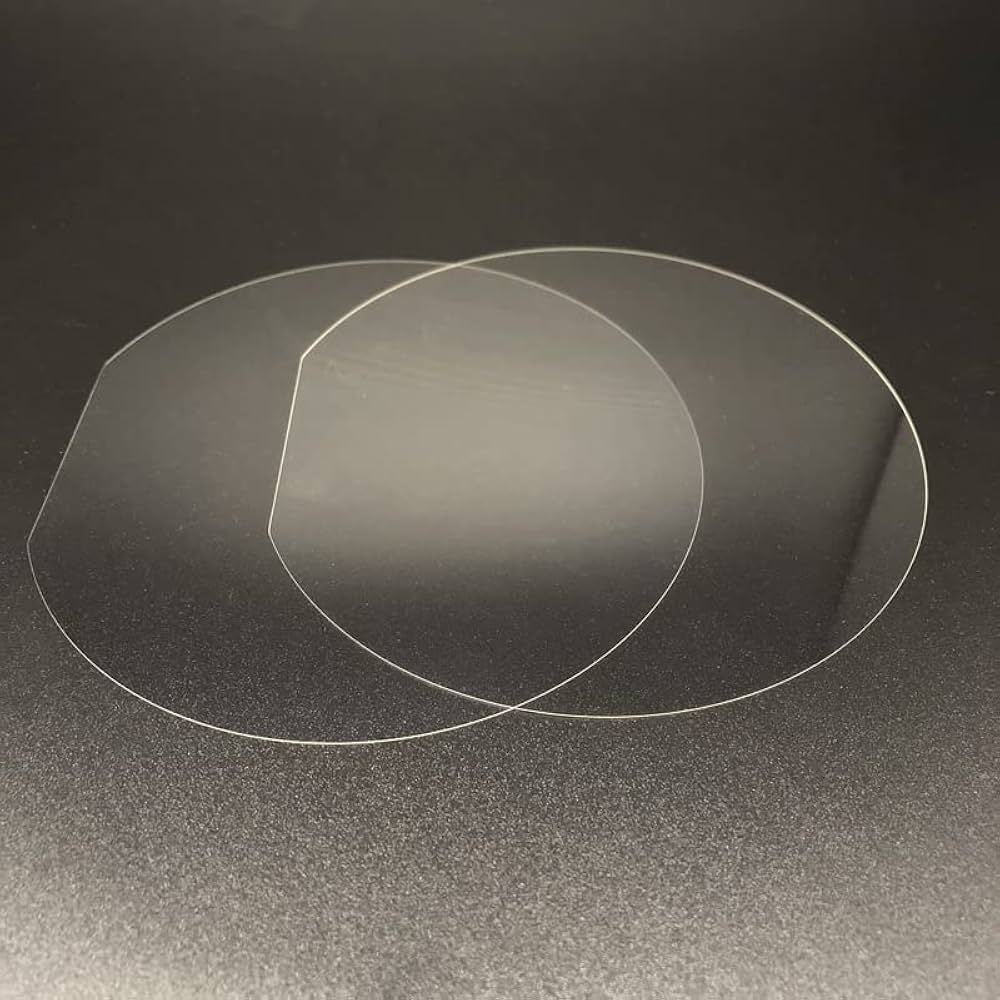
• Precision Manufacturing for Consistent Sealing
Sapphire's benefits appear only when geometry is right. Our process focuses on precision:
✅ Tight tolerance CNC machining for consistent fit and low runout.
✅ Fine lapping and polishing to achieve a smooth, stable sealing face.
✅ Stress-aware fixturing to protect the single-crystal structure during fabrication.
Because sapphire is brittle, it requires expertise. Our teams tune each step to preserve strength while reaching the finish that low-leakage designs demand. The result is a Sapphire Valve Disc For Medical Use that keeps its surface, keeps its shape, and keeps performing.
Implementation, Validation, and Next Steps
When selecting ceramics, match the disc to the operating environment. Consider temperature range, media chemistry, pressure, and cycle count. Sapphire excels where high temperatures, corrosive agents, or high-energy exposure are present. It is a premier “engineering crystal” used for optical windows, semiconductor substrates, and precision instrument protection; those same traits carry over to medical fluid control, where reliability is paramount.
For medical device engineers, the pathway is straightforward. Define the sealing geometry and loading. Specify surface finish and flatness targets. We can advise on edge design to minimize stress risers, and on mounting schemes that protect the single-crystal during assembly. This keeps the focus on performance, not on workaround fixes.
At UPCERA, we build Sapphire Valve Disc For Medical Use solutions to help devices run longer with fewer service stops. Our sapphire's hardness, thermal stability, and chemical inertness work together to reduce wear and preserve sealing over time. That's durability you can design around - and reliability patients can depend on. If your roadmap includes biocompatible sapphire valve components or you're updating an existing platform, we're ready to help.
CTA - Talk with UPCERA
Ready to evaluate sapphire for your next valve? Contact UPCERA for engineering support, sample recommendations, and a rapid manufacturability review.
 English
English 中文
中文